To celebrate the upcoming World Microbiome Day, we’ve compiled seven crazy facts about your resident gut bacteria; from microbial CSI to crapsules, these facts will leave you as mad about the microbiome as we are!
Table of contents
- 1.) The microbiome has been coined the “second genome”
- 2.) Our microbiome is as unique as a fingerprint
- 3.) Gut bacteria can “steal” genes
- 4.) The microbiome educates our developing immune system
- 5.) There are numerous bacterial species yet to be discovered
- 6.) Faecal Microbiota Transplants can treat Clostridium Difficile infections
- 7.) Our microbiota may influence mental health
- Celebrate World Microbiome Day by exploring yours
Whilst we can’t see them with the naked eye, humans play host to trillions of microbes in and on their bodies, collectively known as the microbiome.
The largest and most important community of microbes resides in the human gut, but there is also an oral, skin and vaginal microbiome.
The gut microbiome comprises multiple microorganisms, including eukaryotes, archaea, fungi, viruses and most abundant of all, bacteria.
Humans exist in symbiosis with their resident bacteria, meaning that we mutually benefit each other.
Whilst they need us for a space to live, we rely on them to digest resistant starches, synthesise vitamins and even train the developing immune system.
There is still much we don't know about the microbiome, though it is clear that our gut bacteria are instrumental to human health and represent a new frontier in biomedical science.
In honour of the upcoming World Microbiome Day, we’ve compiled a list of the seven craziest microbiome facts. Without further ado, let's jump in:
The microbiome has been coined the “second genome”
The microbiome refers to the collective genome of all the microorganisms in our body. It might surprise you to learn that bacterial genes dwarf our human genome, numbering in the millions compared to our measly 20,000.
Each of these bacterial genes programs for a specific function, many of which benefit humans and patch up gaps within our genome.
For example, our human genes struggle to digest certain indigestible fibres, but our gut bacteria have just the tools for the job and fill in for our human enzymes.
Researchers call the microbiome our “second genome”, as its genes complement and augment our human ones.
The "second genome" opens up a new frontier in health research and is the subject of numerous ongoing studies, such as the Human Microbiome Project (HMP).
The HMP is an ongoing American initiative seeking to collect a diverse database of microbiome samples.
Similar to the Human Genome Project, the landmark enterprise seeks to help researchers identify microbial markers of health and disease for therapeutic application.
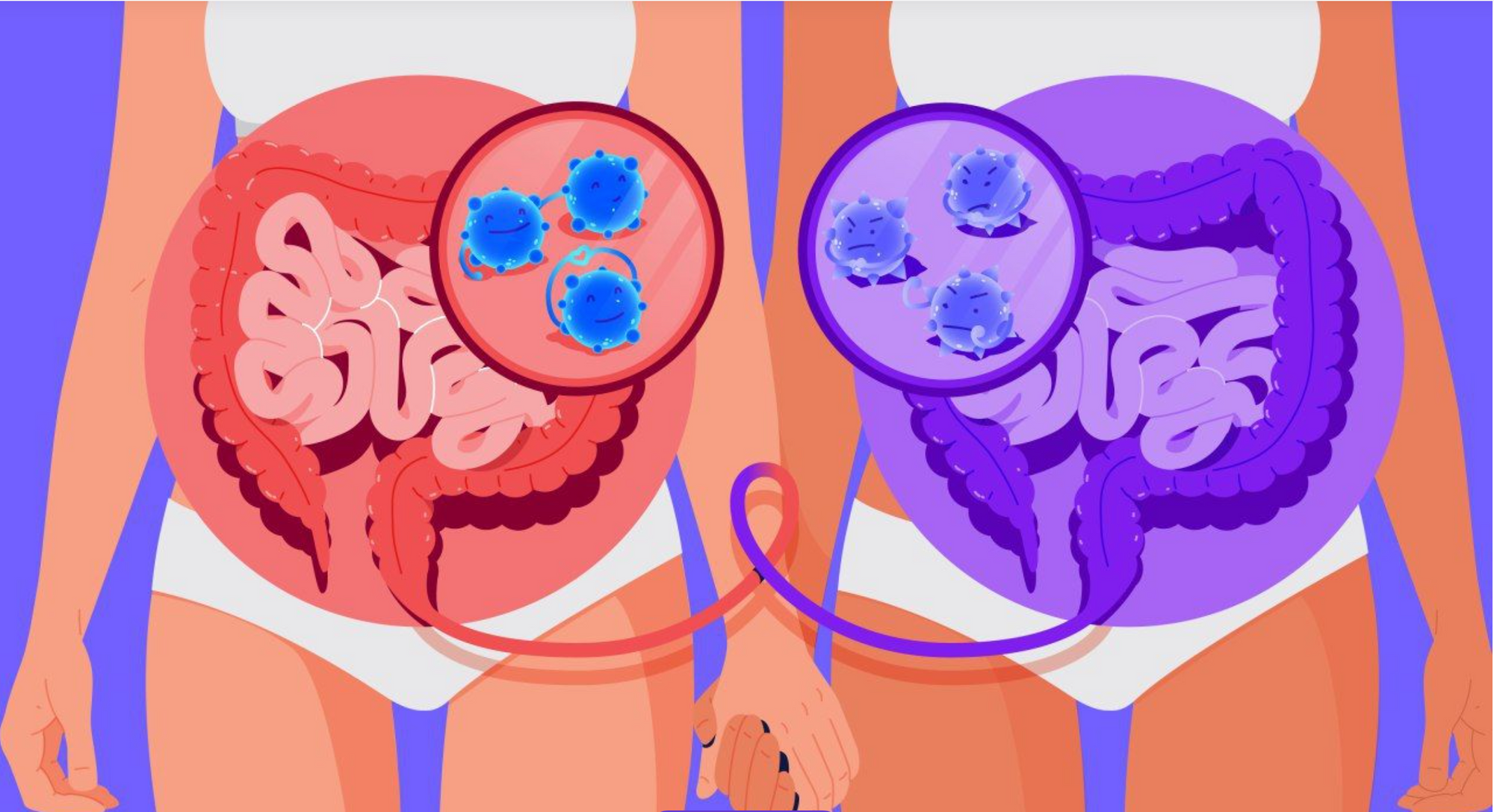
Our microbiome is as unique as a fingerprint

While we share around 99.9% of our DNA with other humans, our microbiomes can be vastly different, sharing as little as 10% similarity!
Take the Macfarlane twins, founders of Gut Stuff- despite being genetically identical, their microbiomes are only 40% similar!
Emerging research suggests that our microbiome profiles may be used to identify us, acting as a microbial fingerprinting system.
In one study, 80% of subjects remained identifiable within a year of having their gut microbiome sampled, although microbial skin trails were shown to be far less accurate and stable.
The technique is unlikely to be adopted for forensic application, mainly because the microbiome is subject to change, and it's unlikely someone would leave their intestinal microbes at a crime scene. However, this didn’t stop CSI Miami from featuring the hypothetical technique in an episode.
The microbiome educates our developing immune system
Immunity is not hard-wired into our genes but acquired during our earliest years. As it turns out, gut bacteria play a crucial role in the programming of the immune system, potentially influencing health outcomes in later life.
From around one to five years old, our gut bacteria train GALT immune cells to tolerate beneficial and commensal bacteria, thereby preventing unnecessary inflammation.
After graduating from microbe middle school, the immune cells are let loose in the body to put their education into practice.
Parallel to this, those raised in close proximity to animals, either through a household pet or livestock, have lower levels of allergies in adulthood.
Researchers hypothesise that diminished microbiome diversity in childhood, whether through hyper cleanliness, antibiotics or bottle-feeding, hinders our immune education.
In short, dysbiosis means that the immune cells experience a smaller sample of microbes during their training. As a result, they are more likely to attack innocent cells accidentally in later life.
On the contrary, a diverse microbiome allows the cells to become skilled at distinguishing between harmful pathogens and harmless bacteria.
Unlike the immune cells trained by a dysbiotic microbiome, these would have received a broad spectrum education.
Gut bacteria may “steal” genes to expand their functions

Not only can our gut bacteria educate our immune cells, but they are also good students, able to adapt and learn new skills.
For example, bifodobacterium and firmicutes species have been observed to share genes with seaweed-eating bacteria, allowing them to better digest nori and wakame.
Interestingly, the seaweed digesting genes are more common in the microbiomes of Asian subjects, a region where sushi is commonly consumed.
Researchers are unsure whether the bacteria acquired these genes over thousands of years or within months, with opinions split both ways.
According to one hypothesis, rapid bacterial gene acquisition may have allowed our ancient ancestors to adapt quickly to new environments. Otherwise, our forebears would have had to wait for genetic mutations to arise at glacial speed.
There are numerous bacterial species yet to be discovered
Over the last two decades, sequencing technologies have allowed researchers to begin cataloguing gut bacteria from around the world, offering new insights into the microbiome.
While great strides have been made in the taxonomy of gut bacteria, there is still lots of work to be done.
For starters, there are only 440,000 microbiome samples publicly available, compared to 20 million human genome sequences. As such, we are still missing large parts of the picture regarding the “second genome”.
Secondly, the samples available are biased in favour of highly developed, industrialised societies, particularly the U.S. and China.
In these places, caesarian section births, hyper cleanliness and antibiotic use are commonplace, all of which can disrupt microbiome diversity.
Interestingly, the few samples from those living in more ancestral ways (pastoral or hunter-gatherer lifestyles) possess more diversity than those living in highly developed societies. Furthermore, they have also been found to contain bacterial species unknown to science!
Who knows what species and bacterial genes we will discover in guts across the globe?
Faecal transplants can re-shape the gut microbiome and cure bacterial infections

Faecal microbiota transplants (FMT) are an ancient treatment dating back 1000 years to Chinese practitioners.
During the procedure, the patient is transplanted with a faecal sample from a “healthy” donor, often via enema or colonoscopy.
Whilst it may sound gross, FMT has proven a safe and highly effective treatment for clostridium difficile infections, boasting a 90% success rate!
Clostridium difficile is a debilitating condition where pathogenic bacteria take over the gut. In many cases, the condition is triggered by heavy antibiotic use.
In 2012, a group of researchers from M.I.T university started a non-profit company called OpenBiome, designed to store faecal samples for those in need of donors.
Our microbiota may influence mental health
The gut and brain have long been known to communicate bidirectionally, both via the vagus nerve and other pathways. As such, the gut is known as “the second brain”. Evidence suggests that the microbiome may also influence mood via the same mechanisms.
Firstly, those with mental health conditions, including depression, bipolar disorder and schizophrenia, often have microbiomes characterised by imbalance and increased intestinal permeability (dysbiosis). By itself, it’s not enough the implicate our gut bugs, but it hints at a link.
Moreover, a pivotal study found that mice raised in a germ-free environment, therefore missing a microbiome, exhibit exaggerated physiological responses to stress.
The mechanisms by which our bacteria may shape mood aren't fully understood, although multiple plausible pathways have been suggested. Firstly, imbalances in the microbiome can increase intestinal permeability and inflammation.
Alongside dysbiosis, numerous mental health conditions are characterised by increased inflammatory markers in the bloodstream, suggesting the two are related.
Additionally, gut bacteria synthesise numerous mood-altering chemicals, including the happiness hormone serotonin.
It is hypothesised that microbiota can transmit neurotransmitters via the vagus nerve, shaping behaviour.
Likewise, researchers suspect some short-chain fatty acids may be neuroactive, offering another mechanism by which microbiota may modulate mood.
Celebrate Microbiome Day by exploring yours
June 27th marks World Microbiome Day, a campaign to celebrate the microbial world and draw attention to the latest research.
This year, celebrate Microbiome day by exploring yours with the Atlas Microbiome Test.
With 22 health reports, you’ll discover the balance and richness of your gut bugs, including how this can impact your overall health.
Based on these results, we’ll send weekly, personalised food recommendations to boost beneficial bacteria, cultivate diversity and harness the power of your microbes!

☝️DISCLAIMER☝This article is for informational purposes only. It is not intended to constitute or be a substitute for professional medical advice, diagnosis, or treatment.