Research suggests that common medications other than antibiotics can alter microbiome composition. Likewise, the microbiome appears to influence drug response. We explore how drugs such as proton pump inhibitors and Metformin impact our gut microbiota and vice versa.
Table of contents
- Antibiotics: gut bacteria are harmed in the taking
- The gut microbiome: a new frontier in health research
- Metformin: a microbially mediated medication
- Proton pump inhibitors: harmless antacids or microbial foe?
- Pharmacomicrobiomics: gut bacteria shape how we respond to medication
- Article summary
Antibiotics: gut bacteria are harmed in the taking
As a result, they inflict collateral damage on probiotics, disrupting the diversity and balance of our gut microbiome.
When used in moderation, the microbiome can bounce back from these disruptions most of the time. However, careless antibiotic use can trigger dysbiosis in the gut and pave the way for pathogenic species to gain a foothold.
Antibiotics are one of the primary triggers for clostridium difficile infections, a pathogenic bacteria that causes chronic diarrhoea.
When antibiotics cull beneficial and commensal species, it leaves a power vacuum that robust harmful bacteria can exploit (because chaos is a ladder!).
The best defence against pathogenic gut bugs is a diverse, balanced microbiome and judicious use of antibiotics. Lastly, If you need to take a repeat course of antibiotics over a prolonged period, consider using postbiotics to restore your gut flora.
The gut microbiome: a new frontier in health research
In recent years, the microbiome has emerged as a distinct “organ” crucial for human health. We now know that our gut microbiota train the immune system, synthesise vitamins, fuel intestinal cells and protect against inflammatory conditions.
Emerging evidence suggests that numerous common medications may unintentionally impact the growth of bacterial strains. It remains unclear how this affects microbiome composition and human health generally, although it raises many questions and possibilities.
Metformin: microbially mediated medication
Metformin is a first-line treatment for type-2 diabetes that works by reducing glucose production in the liver and increasing insulin sensitivity.
In studies on healthy controls, the drug has been demonstrated to alter microbiome composition significantly compared to a placebo.
Furthermore, animal and human studies indicate that the microbiome may mediate the drug’s beneficial effects, including reduced glucose production.
When germ-free mice are transplanted with microbiota from Metformin-treated humans, the rodents exhibit lower blood glucose levels, hinting at direct causation.
The microbiome is also a potential cause of the adverse side-effects commonly reported on Metformin. Clinical studies have shown that as many as one-third of those on Metformin experience diarrhoea, bloating and nausea. Interestingly, all of these are symptoms attributed to increased gas metabolism by butyrate-producing bacteria.
The interrelationship between Metformin and the microbiome is a powerful demonstration that non-antibiotic medications can shape the gut microbiome. It also shows that common medications potentially account for drug side effects and action mechanisms.
Additionally, those with type-2 diabetes often share unique microbiome signatures, previously believed to be either a cause, consequence (or both) of the disease.
These findings highlight the need for researchers to factor in medication-related microbial changes when assessing the microbiome signature of disease states.
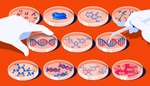
In light of the discovery, a team of researchers at the European Molecular Biology Laboratory explored the effects of over 1000 medications on 40 strains of bacteria. Of the drugs studied, 835 were human-targetted, meaning they weren't antimicrobials.
The paper, published in the prestigious journal Nature, found that 24% of the human-targetted drugs inhibited the growth of at least one bacterial strain during in vitro tests (a test tube).
Of the 230 human-targeted drugs that inhibited bacterial growth, 40 affected ten strains or more. Beneficial species such as Roseburia intestinalis, Eubacterium rectale, and Bacteroides vulgaris were more susceptible to the drug inhibitive effect.
Chemotherapy medication, calcium-channel blockers and antipsychotics inhibited the most significant amount of gut bacteria. Unsurprisingly perhaps, laxatives have also been shown to alter microbiome composition.
Proton pump inhibitors: harmless antacid or microbial foe?

Proton pump inhibitors (PPI’s) are one of the most common drugs worldwide, boasting a favourable safety profile and proving highly effective at treating conditions such as Gastroesophageal reflux disease (GERD).
PPI use has risen rapidly in recent years, with the drug readily available over the counter in the Netherlands and other European countries.
PPI’s reduce acid production in the stomach lining by acting on specific enzymes. Despite their widespread use, PPIs are associated with a reduction in microbiome diversity and an increased risk for clostridium difficile infections.
As an unintended by-product of their antacid effect, they can allow an increased amount of oral bacteria to enter the stomach and gut. It is thought that these foreign microbes upset the balance in the gut, potentially leading to a state of dysbiosis.
Pharmacomicrobiomics: gut bacteria shape how we respond to medication

For example, animal and human studies reveal that the gut microbiome indirectly influences a person’s response to cancer immunotherapy.
In rodent studies, mice treated with commensal bifidobacterium show improved tumour control. Moreover, germ-free mice exhibit a reduced response to anti-tumour drugs, hinting at microbial involvement.
Amazingly, the improved anti-tumour response can be transferred to other mice via faecal transplant, a procedure which reshapes the microbiome composition.
Observations in humans also support the idea that gut bacteria can determine the efficacy of immunotherapy. In one study, patients who received antibiotic treatment before, during or after immunotherapy treatment experienced greater tumour progression than those who hadn't been treated.
As we mentioned earlier, antibiotics are known to disrupt the microbiome and upset our gut ecosystem.
The discovery that gut bacteria can enzymatically change the structure of drugs, thereby impacting their properties, has spawned a new field called pharmacomicrobiomics.
Much like pharmacogenetics (the study of genes and drug response), it seeks to understand how the bacteria in our gut affect our response to medications, including their side effects and efficacy.
Although the field is in its infancy, pharmacomicrobiomics is a promising discipline that could help researchers minimise adverse drug responses and improve the efficacy of medications.
In the future, it is not unfeasible that we could tailor drug responses by changing microbiome composition, whether through prebiotics, probiotics or faecal transplants.
Article summary
- Antibiotics indiscriminately kill bacteria and can disrupt microbiome diversity. This is why antibiotics cause a range of gastrointestinal issues and are considered a primary cause of c.difficile infections
- Emerging research suggests some common medications besides antibiotics may alter microbiome composition, including proton-pump inhibitors, anti-diabetic drugs and statins.
- Drug-related microbiome alterations may partly explain the side effects and action mechanism of common medications.
- Unlike the human genome, the gut microbiome is modifiable, making it an attractive therapeutic target.
- Pharmacomicrobiomics is an emerging field which seeks to control drug response through microbiome changes. Along with pharmacogenetics, it has the potential to drive personalised medicine forward.
☝️DISCLAIMER☝This article is for informational purposes only. It is not intended to constitute or be a substitute for professional medical advice, diagnosis, or treatment.